Home - Techniek - Electronica - Radiotechniek - Radio amateur bladen - QST - Practical battery-back-up power for amateur radio stations 3
A Simple Battery Monitor
Monitoring a battery's electrical performance frequently, or even continuously, Is highly desirable. You should at least monitor the system battery voltage; an ammeter for measuring battery charging current can be informative, too. Preferably, a monitor for battery current and voltage should be located where you'll see it every time you enter the shack. The circuit in Fig A, a simple voltage/current monitor, can indicate load and charging current, and battery voltage.
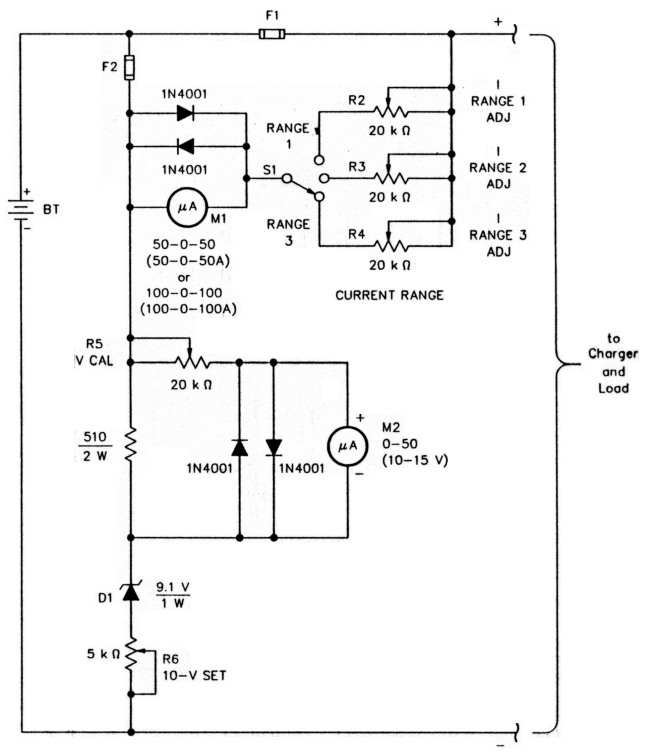
Fig A - Monitoring voltage and current in your back-up-power system Is easier with a specialized voltage and current monitor like this. See the text for how to adjust the V CAL, I RANGE ADJ and 10-V SET trimmers. Component designators contained in the text but not called out below serve to illuminate the text.
BT1 - System battery; see Fig 2 (in Part 1 of this article).
F1 - System fuse; see Fig 2.
F2 - Instrument fuse. 500 µA.
M1 - 50-0-50- or 100-0-100-µA (zero-center) meter; see text,
M2 - 0- to 50-µA meter; see text.
R2 - R5-20-kΩ trimmer pot.
R6 - 5-kΩ trimmer pot.
S1 - Rotary switch.
M1 is a zero-center meter capable of indicating 50 or 100 µA, full-scale. It displays the current flowing into or out of the battery by measuring the voltage drop across F1, which serves as a meter shunt.
The fuse exhibits a resistance of a few hundredths of an ohm; the system load and charging currents cause a voltage drop across It, making M1 deflect. How much M1 deflects depends on the voltage drop and Ml's internal resistance. If the resistance of F1 equals, say, 0.05 tl, and the internal resistance of M1 is, say, 1 kΩ, a current of 20 A through the shunt (a voltage drop of 1.0) drives 1 mA through Ml. That much current would damage a 50- or 100-µA meter, so switchable multipliers (R2, R3 and R4) are included to reduce this current and set M1's full-scale deflection. Adjusting R2 for an indication of 20 µA for a 20-A load current turns a 50-0-50-µA meter into a 50-0-50-A instrument, for instance. R3 can be adjusted for a full-scale current of 5 A. R4 provides a third range for the current monitor. The 1 N4001 s across M1 protect it from overvoltage by limiting the voltage drop across M1 to approximately 0.6 V. The meter is further protected by F2, a 500-µA instrument fuse.
The voltmeter circuit (M2 and its associated components) features an expanded scale; that is, it indicates 10 to 15, instead of 0 to 15, volts. (Like Ml, M2 is protected against overvoltage by 1N4001 diodes.) If possible, use a 0- to 50-µA meter at M2; then, a reading of 50 µA represents 15 V, with 10 µA corresponding to 11 V. The voltmeter circuit works as follows: The full battery voltage appears across the series string consisting of the 510-11 resistor, Dl" and- R6. R6 is adjusted until the voltage drop across D1 and R6 is exactly 10 V, as measured on an accurate digital voltmeter. Then, the voltage drop across the 510-Ω resistor is the difference between the battery voltage and 10 V. R5, V CAL, is then adjusted to make M2's reading agree exactly with that of the digital voltmeter. (For example, if the digital voltmeter indicates a float voltage of 13.50, adjust R5 for a reading of 35 µA on M2.)
With its scale thus expanded, M2's low-end readings are somewhat inaccurate because of Dl's nonlinear conduction curve, but this is Immaterial. Calibrated at your system's normal float voltage, M2 will be accurate at the voltage most critical to proper operation of your system. Any deviation from this voltage can easily be read In tenths of a volt, and estimated 4 hundredths. If you have any cause to doubt M2's calibration accuracy, you can always recheck It with the digital voltmeter. - W4MLE
Now that your back-up power system is in place and operating, you need to keep it there. Back-up-battery monitoring, maintenance and safety round out this three-part series on how to keep your station up when commercial power goes down.
Together, maintenance and monitoring are essential to keep your back-up-power system running efficiently for years. Maintenance includes checking the specific gravity (SG) of the electrolyte in your back-up battery at regular intervals.
Specific gravity is a ratio - the weight of a volume of electrolyte compared to the weight of the same volume of pure water. Pure water has an arbitrary specific gravity of 1.0. The electrolyte is a dilute solution of sulfuric acid in water. Pure sulfuric acid is much heavier than water. Therefore, the more sulfuric acid in the electrolyte, the higher the specific gravity.
Discharging a battery takes sulfuric acid out of the electrolyte and converts it to lead sulfate. Charging a battery reverses this process, restoring acid and raising the specific gravity; when continued charging no longer increases the specific gravity, the cell is fully charged and further charging only electrolyzes water and generates heat. You can estimate the percentage of discharge of a cell quite closely by comparing its specific gravity to its fully charged value.
Typically, lead-calcium float cells use electrolyte with a specific gravity in the range of about 1.22 to 1.28. By contrast, electrolyte in a fully charged automotive battery may read 1.275 or higher. Because of this, measuring back-up cell specific gravity with an automotive hydrometer results in low readings - ''fair'' or "discharged" even for fully charged back-up cells. So, be sure to use a hydrometer calibrated in actual values of specific gravity - not just "good," "fair," and "recharge."
Several factors can make a hydrometer less than ideal for checking cells. For one thing, its calibration may be accurate only near a specified temperature. Cold electrolyte may cause falsely high SG values; warm electrolyte may cause falsely low SG readings. The pointers in some hydrometers tend to stick, giving readings that are hard to interpret. Even a hydrometer using a small glass float in a larger glass cylinder can be a problem: The float may stick to the walls of the cylinder, rather than float free. This destroys the accuracy of the reading. (Take float-hydrometer readings at the meniscus - the lowest point of the concave surface of the liquid in the hydrometer tube. Reading calibrations on the float at the meniscus can be difficult because they're obscured by the liquid surface.)
The state of a cell's charge can also be checked by measuring its terminal voltage several hours after the system charger has been turned off. A reading of 2.1 V per cell is about right, depending on the cell type. With the charger turned on and set to apply 13.5 V to the battery, the terminal voltage across each cell in a lead-calcium float battery should be 2.25.
Cells frequently deviate slightly from this value, however. All six cells in a battery rarely test at exactly the same value. If the values diverge too much, the battery needs an equalizing charge, discussed in Part 2 of this article.
Replacing lost electrolyte
Most cells lose some liquid over time. This is due partly to evaporation, and partly to the decomposition of water into its constituent hydrogen and oxygen gases by electrolysis. Replace lost cell liquid with distilled water, readily obtainable from grocery stores. Don't use tap water; the minerals it contains contaminate the electrolyte and eventually interfere with battery performance.
Occasionally, you may spill some electrolyte from a cell. (If you do, safety, not the battery's state of charge, is your first concern! See below.) In that case, the cell loses sulfuric acid and water. If not much liquid is spilled, it may be replaced by swiping small amounts of electrolyte from other cells in the battery, then bringing all the cells up to full volume with distilled water.
Considerable electrolyte spills must be replaced by new electrolyte made by mixing sulfuric acid with water in the proper proportion to produce electrolyte of the same specific gravity as that of the electrolyte already in the cell. This process involves handling strong, highly corrosive acid that can burn the skin almost instantly and create sores that are painful and very slow to heal. Unless you really know what you're doing, don't mix your own electrolyte; go to a battery shop and get replacement electrolyte of the correct specific gravity.
Safety(14)
Warning: Heavy-duty lead-acid cells are potentially very dangerous. Each cell contains fairly strong sulfuric acid, which can cause injury when in contact with the skin and blindness when in contact with the eyes. Handle the cells with great caution and respect. A 300-Ah float cell contains about three gallons (12 to 13 liters), more or less, of 6-molar sulfuric acid. This means that each healthy, fully-charged cell contains about 15 pounds (6.8 kg) of concentrated sulfuric acid - wicked stuff.
Whenever you handle these cells, as in installing them or moving them, wear liquid-proof safety goggles and acid-resistant rubber (or plastic) gloves. Keep a garden hose handy, with water turned on. Keep a supply of sodium bicarbonate (also known as bicarbonate of soda, or baking soda) at hand to neutralize acid that may get on your skin. Have another person standing by to help if something goes wrong.
If any electrolyte makes contact with your skin or clothing, safety experts15 recommend taking the following measures immediately:
- Hose down the affected part of your body. While you're under the hose, quickly remove and discard any clothing splashed with acid.
- If you get acid on your skin, flood the spill with water immediately, rinse thoroughly for several minutes, then dust the affected area with sodium bicarbonate.
If electrolyte splashes into your eye(s), the recommended immediate treatment is to:
- Immediately flush your eyes with water for at least 15 minutes, including under the lids. Speed in starting the flushing is critical. While your eyes are being flushed, call medical help. A 911 call is appropriate.
Flushing with large quantities of water is also the proper treatment for electrolyte spills on skin. Elderly people and young children's skin is especially vulnerable to acid, but 6-molar electrolyte causes itching and stinging even in healthy young adults within a few seconds of contact. (Broken or irritated skin reacts quicker, and more strongly.) If the electrolyte is flushed away with water and neutralized with baking soda, chances of serious injury are minor.
The polycarbonate-plastic cases of backup batteries and cells are extremely strong and acid-resistant, but they must be treated with respect. A 300-Ah float cell weighs about 85 pounds. If you drop it and its case cracks, you have a very dangerous mess on your hands (or feet)!
Dust and gunk of various kinds tend to accumulate on the cases and must be cleaned off periodically. The best cleaner is a damp cloth or paper towel. But if acid is spilled on the outside of the case, it should be neutralized so it won't damage other materials or injure skin that comes into contact with it. Battery manufacturers recommend a dilute solution of baking soda. This extremely mild alkali neutralizes the acid without damaging the plastic. (Baking soda fizzes because it reacts with the acid to produce sodium sulfate and carbon dioxide gas.) Don't use stronger alkalis, such as lye (sodium hydroxide) or ammonia (ammonium hydroxide) on backup batteries. These chemicals damage the cases, causing cracking that may lead to leaks. Also, don't use organic solvents such as alcohol or carbon tetrachloride.
When batteries go bad
Typically, a healthy new cell can be completely discharged and recharged several hundred times before old age sets in. If the discharges are shallow, the cycle can be repeated many more times - perhaps 600 to 800 times. A cell is considered dead when it can deliver no more than about 30 percent of its original capacity after being fully charged.(16)
A cell may die for various reasons. One of them is poisoning by foreign substances, such as copper or iron ions from foreign materials. Another is a gradual buildup of lead sulfate that refuses to convert back to lead peroxide and spongy lead. This happens because lead sulfate tends to form larger crystals over time, and the larger particles don't react as readily to the charging current. Another reason for cell failure is that solid materials, slowly flaking off the plates, fill the small spaces between plates, causing internal short circuits or blocking circulation of electrolyte.
Disposing of unusable cells
Batteriesare considered by environmental agencies to be hazardous materials. Don't leave discarded units lying around the yard or send them off to the city dump. Safety experts recommend the following procedure to render back-up batteries harmless:
- Take the safety precautions mentioned earlier. This includes wearing the proper protective gear, and keeping a running hose, baking soda and an assistant nearby.
- Dump the electrolyte into a corrosion-proof container. A large plastic bucket works fine. Pour as much liquid out of the battery as possible. (Careful: Back-up cells and batteries are heavy. Lift them safely. Don't splash electrolyte on yourself or your clothing!)
- Using a plastic scoop, add small quantities of slaked lime (hardware and garden-supply stores sell it) to the acid in the bucket while stirring the mixture vigorously with a wooden or plastic tool, like an old broom handle. The resulting chemical reaction generates a great deal of heat! Do not just dump lime into the liquid! (Danger: Like the electrolyte, slaked lime is highly caustic. Do not touch it or breath its dust.)
- After the lime stops reacting with the acid, add a teaspoonful or two of baking soda to the mixture. If it fizzes, some acid remains. Continue to add lime until baking soda no longer fizzes.
- The resulting mess in the bucket consists mainly of water and calcium sulfate - the material of plaster of paris and gypsum. This mixture is harmless and may be disposed of by washing it down the drain or putting it out in the garbage, enclosed in plastic bags, for sanitation pickup.
Discarded cell carcasses, with lead plates, can sometimes be sold to salvage dealers. Dealers may take cells that still contain electrolyte, but check before you transport the cells: They're messy and dangerous to move.
Summary
Back-up power for Amateur Radio stations is useful, and need not be expensive. Heavy-duty batteries intended for float service are available to do this job well. A battery-back-up-power system may be just what you need to keep your station going when emergencies arise and commercial power fails.
Notes
- Material Safety Data Sheet No. 9, Revision B, Oct 1980, Corporate Research & Development, Schenectady, NY 12305.
- Material Safety Data Sheet, Corporate Research and Development, Schenectady, NY 12305, 1980 by General Electric Company.
- C&D Batteries, Stationary Battery Installation and Operating Instructions, C&D Batteries, 3043 Walton Rd, Plymouth Meeting, PA 19462.
Selected Bibliography
- C. Chapman, P. Chapman, and A. Lewison, "Amateur Use of Solar Electric Power," Part 1, QST, Oct 1982, pp 11-14; Part 2, QST, Nov 1982, pp 30-34.
- "Globe Gel Cell Rechargeable Batteries in Standby and Portable Power Applications," ('hanging Manual, Globe Battery Division, 5757 N Green Bay Ave, PO Box 591, Milwaukee, WI 53201.
- D. DeMaw, "Solar-Electric Power and the Amateur," QST, Aug 1977, pp 24-27.
- D. DeMaw, "Solar-Electric-Power Update," Technical Correspondence, QST, Jan 1982, p 46.
- J. Halliday, "Solar Powering a Ham Station," QST, Aug 1980, pp 11-12.
- A. Haynes, "QRZ Sunshine," 73, Jan 1981, pp 114-118.
- G. Hopkins, "A Deep-Cycle Battery as an Emergency Power Source," Hints and Kinks, QST, Mar 1988, p 41.
- L. McCoy, "The Encon Solar Array, a Study in Solar Power," CQ, Apr 1983, pp 48-50.
- M. McNaughton, "My Experience with Solar Power," Technical Correspondence, QST, Jan 1981, p 42.
- M. Mideke, "Alternative Energy - An Overview of Options and Requirements," Part 1: QST, Sep 1987, pp 17-21; Part 2, QST, Oct 1987, pp 19-23.
- E. Noll, "Solar Energy," Circuits and Techniques, ham radio, Jul 1974, pp 54-58.
- E. Noll, "W3FQJ Goes Solar Power," Circuits and Techniques, ham radio, Nov 1974, pp 52-57.
- E. Noll, "36-Volt Solar Power Source," ham radio, Jan 1977, pp 54-56.
- W. Schreiber, "Complete Solar Power for Your Ham Station," ham radio, Dec 1984, pp 14-16, 19, 20-23.
- G. Thurston, "Designing Low Voltage Power Supplies," ham radio, Mar 1985, pp 46-49, 51-54, 56-59. (Note: This article contains several errors: [1] Fig 9A, p 57, shows the base of driver transistor Q3 connected to the bases of the pass transistors, Q1 and Q2. Delete this connection and connect Q2's base to R7 and the Q3's collector. [2] In the parts list on p 57, C5 is 120,000 µF or greater, 50V [not 0.1 to 2.0 µF, 50 kΩ]. [3] The diode in Fig 6, p 51, is reversed. Its cathode (banded end) should be connected to the 78XX input. [4] Also on p 51, below the equation in the right-hand column, change the sentence to read "...E = 1.4 volts.")
W4MLE, George L. Thurston III.